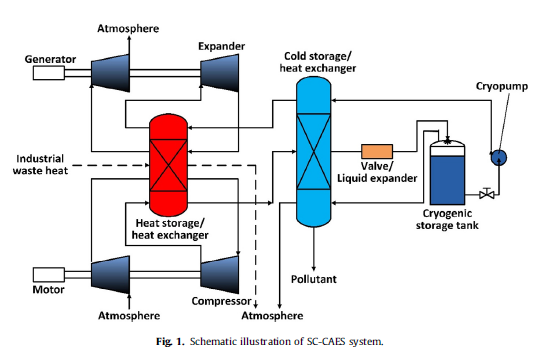
A novel supercritical compressed air energy storage (SC-CAES) system is proposed by our team to solve the problems of conventional CAES. The system eliminates the dependence on fossil fuel and large gas storage cavern, as well as possesses the advantages of high efficiency by employing the special properties of supercritical air, which is significant for the development of electrical energy storage. The thermodynamic model of the SC-CAES system is built, and the thermodynamic characters are revealed. Through the exergy analysis of the system, the processes of the larger exergy destruction include compression, expansion, cold storage/heat exchange and throttle. Furthermore, sensitivity analysis shows that there is an optimal energy releasing pressure to make the system achieve the highest efficiency when energy storage pressure is constant. The efficiency of SC-CAES is expected to reach about 67.41% when energy storage pressure and energy releasing pressure are 120 bar and 95.01 bar, respectively. At the same time, the energy density is 18 times larger than that of conventional CAES. Sensitivity analysis also shows the change laws of system efficiency varying with other basic system parameters. The study provides support for the design and engineering of SC-CAES.
Conclusions
Targeting the problems of conventional CAES, a novel CAES named SC-CAES system by employing the special properties of supercritical air is proposed. The new system eliminates the dependence on fossil fuel and large gas-storage cavern, as well as possesses the advantages of high efficiency and high energy density. In order to better understand and improve the system, thermodynamic model of the SC-CAES system is built, and the thermodynamic characters are revealed. The study provides a feasible pathway for development of CAES system. The efficiency of SC-CAES system can reach 67.41%, which is higher above 13 percentage points than that of conventional CAES. Meanwhile, the energy densities of SC-CAES can reach 3.46× 105kJ/m3, which is18 times larger than that of conventional CAES. Exergy destruction distribution of SC-CAES system is analysed. The compressors, expanders, cold storage/heat exchanger, valve and reheaters bring larger exergy destruction, thereinto, the compressors bring the largest exergy destruction, and the expanders bring second largest exergy destruction. The sum of the two exergy destruction accounts for 45–58% of the total exergy destruction. In addition, exergy destruction of the cold storage/heat exchanger and valve also accounts for larger ratio of total exergy destruction. Through the exergy analysis, it can be known that the exergy destruction of depressurization in the liquid expander is far smaller than that in the throttle valve. This leads to the efficiency of LESC-CAES be higher 6.9 percentage points than that of V-SC-CAES.
Thermodynamic laws of the SC-CAES system are revealed. The efficiency of the system firstly raises and then falls with the increasing of energy releasing pressure when energy storage pressure is constant. That is, the curve of efficiency as the function of energy storage and releasing pressure have the highest point. The highest point is a turning point because the heat transfer of cold storage/heat exchanger crosses the critical temperature of air. The system efficiency linearly varies with compressor efficiency, expander efficiency, pressure loss of each intercooler and pressure loss of each reheater. A turning point exists in the curves of system efficiency varying with MTDCS/HE, cryopump efficiency, and liquid expander efficiency.
The results have been published on Energy Conversion and Management 115 (2016) 167–177.