|
|
| Investigation of Corrosion Characteristics of High-Sodium High Chlorine Lignite during Circulating Fluidized Bed Combustion |
|
| |
| Author: Qi Xiaobin
| |
| Text Size:
| 2018-08-16 |
Corrosion experiments were conducted in a 0.4 T/D circulating fluidized bed (CFB) test rig with the high-sodium high-chlorine lignite as fuel to investigate the corrosion phenomena and mechanisms through the air-cooled probes in furnace and tails, respectively. Three alloy materials involving four metal elements (Al, Cr, Ni, and Fe) were used to compare their anticorrosion characteristics with the thermodynamic equilibrium calculation software Factsage 6.1 as an auxiliary tool. Experimental results indicate that the probes exposed to the high-temperature flue gas in the furnace suffered severe corrosion, which was primarily caused by gaseous corrosive media, including HCl (g), NaCl (g), and Cl2 (g), while the probes in tails underwent the almost negligible corrosion arising from the deposited NaCl crystal on the top surface of the probe. The protective oxidation scale formed on the outer surface of alloy was the key to corrosion resistance rather than the internal metal with high quality. Despite different alloy elements matching different corrosive environments for its optimal anticorrosion, the Al2O3 was found most effective to resist the chlorine-induced corrosion in this study. In accordance with the Gibbs free-energy change (Delta G) of corrosion reactions, the increasing temperature failed to reduce the difficulty of corrosion in essence. The accelerated corrosion at a higher temperature was mainly ascribed to the improvement of the diffusion of the gaseous corrosive species and gaseous reaction products (metal chlorides). At a low-level wall temperature, to a certain extent the influence of gas temperature and alloy materials on corrosion can be ignored. Conclusions: In this paper, the corrosion experiments were conducted on a 0.4 T/D CFB test rig with the high-sodium high-chlorine lignite as fuel to investigate the phenomena and mechanisms through the air-cooled probes in furnace and tails, respectively. The main conclusions are listed below. (1) In the furnace, the probe composed of 06Cr19Ni10, after the exposure to the gas temperature of ~940°C and wall temperature of ~525°C for 8 h suffered obvious corrosion. This corrosion was mainly caused by the gaseous chlorine-bearing species released from the raw coal at high temperature, including HCl (g), NaCl (g), and Cl2 (g). However, the similar probe was free of corrosion due to its low wall temperature (200-250°C), except for the slight corrosion caused by the deposited NaCl crystal in the interface between the alloy and the deposited ash. (2) The protective oxidation scale caused by O2 was formed on the top surface of alloy before corrosion. The priority of four alloy elements (Al, Cr, Ni, and Fe) oxidized by O2 was obtained via calculation, and they obeyed the order of Al > Cr > Fe (from Fe to ferrous Fe) > Ni > Fe (from ferrous Fe to ferric Fe). The oxidation scale was the key to anticorrosion because their corrosion reactions with positive Delta G can not occur spontaneously. The computing results suggest that the ability of metallic oxides to resist corrosion follows the order of Al2O3 > Cr2O3 > NiO > Fe2O3 > FeO. Once the oxidation scale is destroyed, the internal metallic matrix will suffer the ruinous corrosion. This means a high quality is not necessary for the internal metal, and the key to anticorrosion is the outer alloy material. In these experiments, the ordinary alloy material sprayed with the anticorrosion Al2O3 coating seems the optimal case. (3) At a low-level wall temperature, the influence of gas temperature and alloy materials on corrosion was little. Although an increasing temperature accelerated corrosion in this study, it was not achieved by reducing the difficulty of corrosion in essence but improving the diffusion of the gaseous corrosive species and gaseous reaction products (metal chlorides). Besides, the dew point corrosion should be paid attention at low wall/gas temperature. The results have been published on Energy Fuels 2017, 31, 13627-13638. 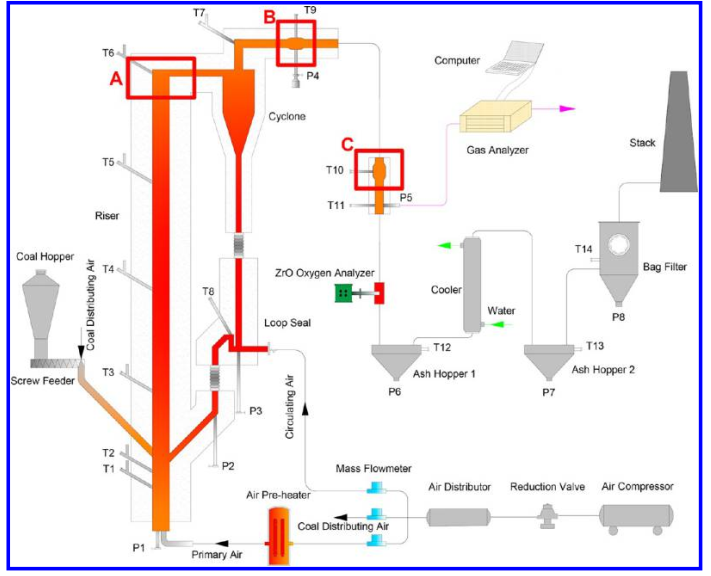
Figure 1 Schematic diagram of the CFB test system |
|